Invizius's Pioneering Dialysis Product H-Guard® Completes First In-Human Clinical Study
The study investigated the safety of H-Guard® in patients undergoing haemodialysis who are vulnerable to dialysis-induced immune activation. H-Guard® is flushed through the dialysis machine, coating all blood exposed surfaces prior to treatment.
Patients received a single treatment of H-Guard® with pre and post treatment evaluations demonstrating its safety and tolerability.
The next stage is the Phase2b study for Acute Kidney Injury (AKI) where the Continuous Renal Replacement Therapy (CRRT) filter in an Intensive Care Unit (ICU) is coated with H-Guard®.
Dr Magnus Nicolson, CEO of Invizius, said: "It has been a pleasure to work with Professor Mitra and his team to demonstrate the safety of H-Guard® on dialysis patients. This is an important milestone for H-Guard® and Invizius, allowing us to progress into a larger efficacy study showing the clinical benefits of controlling complement activation in CRRT and a number of extracorporeal systems."
Professor Sandip Mitra, Consultant Nephrologist and Professor of Renal Medicine at Manchester University NHS Foundation Trust, commented: "As our aim is to improve the outcomes of patients on dialysis, we are very encouraged by the results of the H-Guard® trial. This week at the ERA in Stockholm, Dr Ilyas Duha will be presenting data from Invizius sponsored trial CompAct-HD. This is the largest kinetic analysis of complement activation in routine haemodialysis (HD) ever undertaken in UK. Our results indicate patient specific complement activation associated with inflammatory response during routine haemodialysis. Reduction in complement activation in HD may yield significant clinical outcome benefits."
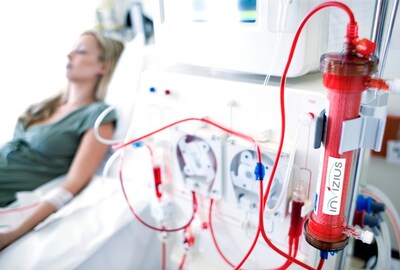
H-Guard® is a potent anti-inflammatory and anti-coagulatory, second generation, complement regulator which coats the dialysis filter and tubing during the priming process.
Invizius managed the trial with TCRSolutions.
Media enquiries:
Lorna Gardner Media House
[email protected]
+44 (0)7813 193618
![]() View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/inviziuss-pioneering-dialysis-product-h-guard-completes-first-in-human-clinical-study-302152886.html
View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/inviziuss-pioneering-dialysis-product-h-guard-completes-first-in-human-clinical-study-302152886.html





